Recipe for an improved lithium–sulfur battery cathode
Motivated by the ganache centre, hard chocolate coating and hazelnut sprinkles of a truffle, scientists in the US have mimicked this design to form a layered lithium–sulfur battery cathode with a sulfur–carbon centre encapsulated in a multi-layered polymer shell sprinkled with carbon.
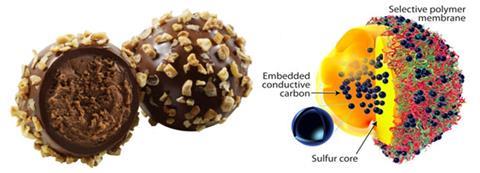
‘The most challenging part of this work was developing a procedure to introduce carbon inside the sulfur core,’ says John Muldoon, who led the team from the Toyota Research Institute of North America. Adding conductive carbon into the core boosts the conductivity, providing a much needed lift for insulating sulfur, but too much carbon reduces the sulfur content. Sulfur is a high capacity cathode material, but its use has drawbacks.
During battery cycling, sulfur reduces to polysulfides that dissolve in electrolyte, which carries them away from the cathode. This loss of active sulfur affects the battery performance and lifetime. In the truffle-inspired core, the team partnered sulfur with hollow carbon structures that allow space for polysulfides to congregate. To stabilise this sulfur–carbon unit, they used a thin, conductive polymer layer to maintain its shape, allow electrolyte to pass to the sulfur core, and act as a foundation layer. But, this thin polymer layer cannot trap polysulfides in the cathode material on its own. To reinforce the thin foundation and prevent the polysulfides from escaping, a flexible, conductive multi-layered polymer outer membrane was added.
The layered outer membrane is ion-selective; singly-charged lithium salts can move easily through the membrane whereas bulky doubly-charged polysulfide salts cannot. By piling up layers of polymers, some of them crosslink due to the pairing of some charged units in the membrane, but some do not pair, creating a maze of structural defects for ions to negotiate. Carbon nanospheres decorating the outer membrane surface further boost the cathode’s conductivity.
The overall result is a high performance, stable, cathode material with 65% sulfur loading and minimal carbon additives that can operate at a high rate for over 500 cycles with nearly 100% coulombic efficiency. Even after stopping the battery cycling for 2 days, the team did not observe any loss in capacity when cycling resumed.
‘Even though the synthesis of a truffle-like powder with high sulfur loading is a little complicated in comparison with traditional sulfur–carbon materials, not only the surface chemistry, but also the bulk structure control of cathode materials is important,’ explains Pengjian Zuo, an expert in energy storage materials from Harbin Institute of Technology, China. Zuo thinks that a layered design is crucial for influencing lithium–sulfur battery properties.
References
This article is free to access until 18 January 2016











No comments yet