Classic multicomponent reaction finally gets chiral touch
59 years after its discovery, the Ugi reaction becomes enantioselective with the help of a chiral phosphoric acid
More than half a century after Ivar Karl Ugi developed his classic four-component reaction, chemists have for the first time found a catalyst that makes the process asymmetric.
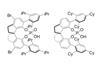
Ugi’s reaction combines a carboxylic acid, an amine, an isocyanide, and an aldehyde or ketone, to make an α-acylaminoamide. Ugi was interested in reactions that, using simple starting materials, can make large chemical libraries. The only problem with this reaction: the tertiary carbon centre it creates is racemic.
A team of chemists led by Bin Tan, from China’s Southern University of Science and Technology, and Kendall Houk, from the University of California Los Angeles, US, have found a set of phosphoric acid catalysts that, for the first time, makes the Ugi reaction churn out products with up to 94% enantiomeric excess.