Clever carbene synthesis replaces notoriously hazardous chemistry
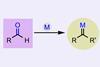
Reactive carbon compounds can now be made from aldehydes instead of explosive or unstable reagents
Aldehydes can now be used instead of explosive diazo compounds or unstable dihalides to make carbene precursors for over 10 different reaction types. ‘We invented a new, safer way to make carbenes that enables all the unique, valuable reactivity of these compounds without the extra “bang” of unstabilised diazo reagents,’ says study leader David Nagib from Ohio State University in the US.