Clippanes join rotaxanes and catenanes in mechanically interlocked molecule family
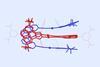
Keck-clip molecules consist of two entangled gold–carbene metallotweezers
A new member has joined the family of mechanically interlocked molecules. Its discoverers dubbed them clippanes since their chemical structure resembles that of two intertwined Keck clips.
