Cocrystal intermediate is first for mechanochemistry
In situ Raman spectroscopy reveals how reactants align prior to forming new bond
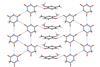
Scientists in Europe have unveiled a cocrystal intermediate that forms during milling in a solid-state Knoevenagel condensation reaction. The cocrystal, which orientates the reactants so they can form a new carbon–carbon double bond, is the first cocrystal intermediate to be observed during a mechanochemical reaction.
