Complex amines made easy (and cheap)
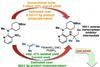
Iron-catalysed cross-coupling brings together nitroarenes and olefins in a single step in boon for drug makers

Iron-catalysed cross-coupling brings together nitroarenes and olefins in a single step in boon for drug makers