Computational study predicts new high-pressure polymorph of Roy
Conformational energy-corrected DFT combined with crystal structure prediction leads to first crystal energy landscape for Roy that agrees with experimental evidence
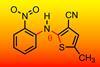
The molecule 5-methyl-2-[(2-nitrophenyl)amino]-3-thiophenecarbonitrile, commonly called Roy (red–orange–yellow) because of the colours of its crystals, is known as the organic compound with the most polymorphs. Now, Gregory Beran and collaborators from the University of California Riverside, US, and Imperial College London, UK, have developed the first crystal energy landscape for Roy that agrees with experimental evidence and concluded that researchers have already found the most stable Roy polymorphs.