Debate surrounding amorphous selenium’s structure rumbles on
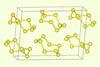
New evidence suggests that amorphous selenium forms eight-membered rings, but some researchers believe the results are inconclusive
Researchers in China have reported new evidence that they believe finally resolves a long-standing debate over the structure of amorphous selenium. Their structural analysis suggests that vapour-deposited samples form eight-membered rings rather than chains of atoms, but other researchers remain unconvinced by these latest results.
