DNA-coated nanoparticles take crystal engineering into the diamond league
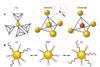
Self-assembling superlattices built with DNA could usher in era of materials on demand

Self-assembling superlattices built with DNA could usher in era of materials on demand