DNA helix has chiral water ‘spine’
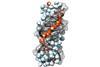
Spectroscopy reveals how water molecules form a chiral superstructure in DNA’s minor groove
Since its discovery in 1953, the helical structure of DNA has become iconic. Researchers in the US have now found that this corkscrew shape isn’t just confined to the DNA molecule itself, however. They say that the DNA double helix also imposes a chiral twist on the arrangement of water molecules that surround it in solution.
