Doubly negative dianions can form π hole bonds too
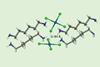
Researchers report an attractive interaction between two doubly negative dianions for the first time
Scientists have uncovered an unusual attractive interaction between two tetrachloridopalladate (PdCl42–) centres. It’s the first time a π hole bond has been reported for two doubly negative dianions.