A new electrochemical method forms different products depending on the shape of the electrical signal that is applied. By tailoring various waveform parameters, researchers in China directed a catalytic reaction to make either a cross-coupling or difunctionalisation product with complete selectivity.
Electrosynthesis – using electricity to generate compounds in an electrochemical cell – is an increasingly popular green alternative to traditional chemical methods. Using electrons in place of conventional reagents for redox transformations eliminates a significant amount of chemical waste, while the tunability of both the voltage and current provides an extra measure of control over the reaction outcome.
Historically, direct current (DC) – which maintains a steady voltage over time – has dominated this field, powering important industrial reactions such as the chloralkali process and water electrolysis. In contrast, alternating current (AC) electrosynthesis is relatively underexplored as the rapidly changing current and voltage introduce many additional variables. However, these additional variables also offer the possibility for even greater control over a reaction’s outcome.
It’s this control that Aiwen Lei and his team at Wuhan University in China have now exploited with a new technique that they call ‘programmed AC electrosynthesis’. By varying different AC parameters during copper-catalysed C–H bond transformations, the researchers can generate either alkynylation or difunctionalisation products from a single set of starting materials.
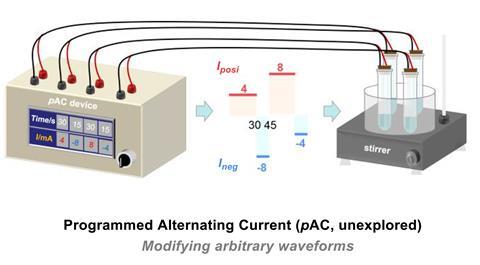
The precise mechanism of a particular metal-catalysed reaction depends on a combination of factors including the oxidation state of the metal and the availability of electrons for oxidation and reduction processes. Using tailored electrical waveforms, Lei could promote one catalytic pathway over the competing reaction, favouring the formation of a particular active species to direct the reaction towards a specific product.
‘For the C–H alkynylation, we have three reductions and three oxidations. For the difunctionalisation we have two reductions and two oxidations, so the fundamental difference is how many electrons are being oxidised or reduced,’ explains Long Luo, an organic electrochemist at the University of Utah, US, who was not involved in the project. ‘These [electron transfers] are sequential so by tuning the wave shapes, you can selectively stop the reaction at the second reduction process and control the reaction pathway.’
Recent AC electrosyntheses have used regular wave shapes such as the sine wave form of mains electricity and the square waves used in rapidly alternating polarity reactions. However, by investigating the role of other AC parameters such as current intensity, frequency, and duty cycle, Lei’s team could tailor the wave shape more closely to a specific reaction mechanism, boosting both the selectivity and yield in the process. ‘Different amounts of current affect the driving force behind the reaction and the frequency controls the time parameter,’ explains Luo. ‘The duty cycle is how symmetric the wave is – how long it has oxidation or reduction. These parameters all control the waveform, which you can play around with until you get the best fit to your reaction.’
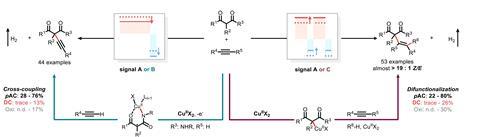
The team optimised these conditions for the two competing pathways, identifying the unconventional wave shapes that promoted each mechanism. They then developed a simple device to generate irregular waveforms from programmed parameters, theoretically permitting these conditions to be easily tuned for different reactions in future.
The ease and scope of the method has already impressed other researchers who say it is a promising demonstration of the potential of alternating current for synthesis. ‘Lei and co-workers have done an excellent job providing a mechanistic rationale that explains the observed reactivity under AC,’ says David Cantillo, an organic electrochemist at the University of Queensland, Australia. ‘AC electrolysis can enable transformations that perform poorly under DC and adoption of this technology will significantly grow as additional research on the effect of the AC parameters on the reactivity at the molecular level is conducted.’
‘It’s beautiful work and they demonstrate huge scope with reasonable yields,’ says Luo. ‘We still have room for deeper understanding [in the field]. Future approaches should establish a theoretical framework and tools for us to rationally design conditions.’
References
L Zeng et al, Science, 2024, DOI: 10.1126/science.ado0875













No comments yet