First four-twist aromatic molecule made
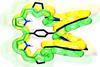
A di-palladium complex with 54 π electrons is the first quadruply twisted aromatic molecule
The first quadruply twisted aromatic molecule has been made by chemists in Japan. Its 54 π electrons are packed into a ring that has four 180° twists.