Actinium’s coordination behaviour is significantly different from its homologue lanthanum, the first single crystal x-ray structure for the element has shown in work that could help pave the way for new cancer therapies.
Despite having been discovered in 1899, actinium remains one of the most mysterious elements – so much so that even its position on the periodic table and whether it should sit in group 3 is still the subject of intense debate. Today, its coordination chemistry is based on work from 1950, which may not be accurate.
However, there is renewed interest in actinium due to its potential as an anti-cancer therapy. This therapy uses the radioactive isotope actinium-225, which is transported through the body to the targeted area. As actinium-225 decays, it emits alpha particles, which then kills cancer cells with minimal toxicity to the patient.
Before this therapy can be developed further, chemists need to better understand the element’s fundamental chemistry and how actinium is likely to behave in our bodies. Unfortunately, as actinium is highly radioactive and unstable, only a few groups in the world can investigate its properties. Instead, researchers typically perform surrogate experiments, using its rare-earth homologue lanthanum, or heavier actinides believed to behave in a similar way, such as americium or curium.
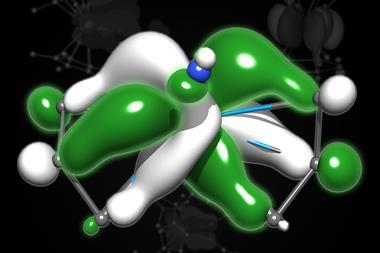
But this approach has its limits, as a team led by Rebecca Abergel at Lawrence Berkeley National Laboratory, US, has shown. Using only 5µg of the longer-lived isotope actinium-227, the team trapped Ac3+ ions using eight ligands to isolate it in a protein scaffold. This allowed the team to investigate its coordination behaviour and investigate the complex’s structure using x-ray crystallography for the first time.
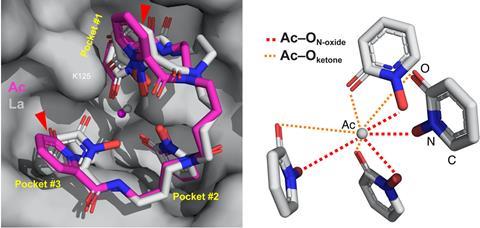
‘We see similarities in the way it forms complexes [compared with lanthanum and other actinides],’ explains Abergel. ‘It resembles it, but the devil’s in the detail … at a very high level you can group the f-block elements, but there are very specific features that will impact the chemistry.’
Rather than the longer interatomic distances expected from actinium’s large ionic radius, the team observed shorter contacts with the surrounding amino acids. ‘It is changing the coordination features in the complex,’ Abergel says. ‘That would change its behaviour in a complex biological system [such as the body]. What this tells us is that we really need to keep looking for those features, so we can leverage them to make more stable complexes.’
The hope is that, with this new coordination data, better chelators can be designed that will allow actinium therapies to take the next leap forward as an anti-cancer therapy. It also, as the group notes, provides valuable information in understanding periodicity across the actinide series and helps feed into the debate as to how the lanthanides and actinides sit on the periodic table.
Thomas Albrecht-Schoenzart, an actinide chemist at the Colorado School of Mines, US, welcomed the ‘key information’ the research provided for future anticancer therapies. ‘Moreover, the differences between its binding and that of La3+ underscores that, in many cases, lanthanides are not adequate analogues of actinides.’
References
JN Wacker et al, Nat. Commun., 2024, 15, 5741 (DOI: 10.1038/s41467-024-50017-5)













No comments yet