Gallium helps sneak antibiotic payload into bacterial cells
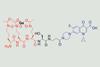
Compound is more potent than parent drug ciprofloxacin against Staphylococcus aureus
Scientists in the US have developed a compound that exploits bacteria’s need for iron to trick them into transporting an antibiotic agent into their cells via their iron uptake and metabolism pathway. Galbofloxacin contains gallium, which ‘looks like iron but cannot be used by the bacteria,’ says PhD student Apurva Pandey, who led the research in Eszter Boros’s group at Stony Brook University. ‘We essentially have a combination therapy, a dual approach of having the gallium present and the antibiotic [meaning] we can go after two mechanisms at once,’ explains Boros.