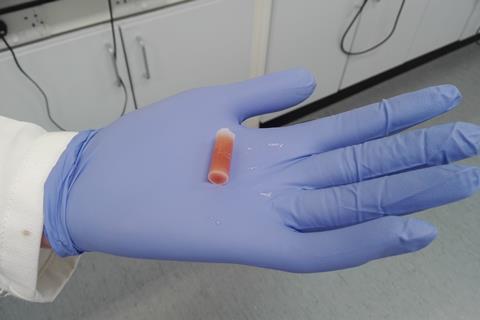
Low-cost gels have improved the stability of organolithium reagents, making manipulation simple and safe. Thanks to this innovative encapsulation method, these reactive compounds – usually sensitive to moisture and air – survive storage under ambient conditions for over 25 days. Moreover, these gel capsules work with other organometallic reagents, such as Grignards, hinting at additional attractive applications protecting pyrophoric compounds and hazardous chemicals.
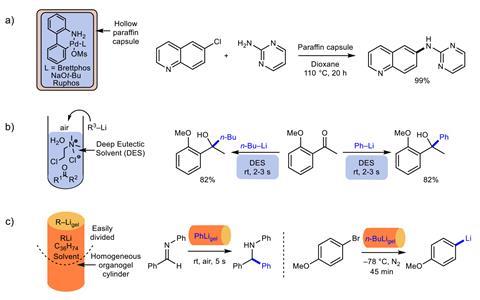
Organic chemists have used organolithium reagents for over a century. Versatile and highly reactive, these organometallic species have become a fundamental tool for synthesis across different disciplines. However, handling them presents problems – organolithiums often need controlled conditions, such as inert atmospheres and cryogenic temperatures. ‘For many years, I had seen [my colleague] Peter O’Brien working with organolithiums, and had been impressed by the high levels of technical skill involved,’ explains David Smith, from the University of York, who co-led this study. ‘After attending a fascinating seminar from Eva Hevia, [who had used deep eutectic solvents] to stabilise organolithium reagents, I wondered whether we could do something similar with our gels,’ he says.
Gels are three-dimensional polymers. In contact with solvents, they become soft, jelly-like materials that retain their cross-linked structure, offering different functionalities. In this case, the team used a cheap hexatriacontane (C36H74) organogel. ‘We took a standard solution of the organolithium reagent in a mould, and added the gelator,’ explains Smith. After gentle heating and cooling, the gel is ready to unmould and use. Among the advantages, researchers can cut the organolithium gels into smaller pieces with scissors or razorblades, which could speed up screening studies. ‘The homogeneous distribution of the organolithium within the gel simply results from the [production] process,’ says Smith. ‘[Scientists] elsewhere have reproduced the methods, and they found the gels stable and easy to handle.’
‘It’s just a spectacular study,’ says Eva Hevia, an expert in organometallic chemistry at the University of Bern, Switzerland. ‘Organolithiums [usually] need vacuum lines, dry solvents and expert handling,’ she explains. ‘The researchers at York challenge conventional wisdom, showcasing that advances and new ideas in chemistry often come from cross collaboration between different areas.’
Although Hevia had previously developed other methods to stabilise organolithiums, she says that these new gels ‘open a treasure chest of opportunities’. Researchers have showcased their versatility carrying out everyday reactions, common in both academic and industrial laboratories around the world. ‘Moreover, the gels possess a striking stability, reaching really high yields after days exposed to air and moisture,’ she says. ‘Even after directly dropping the gels into water, the reactions works – it’s impressive.’
Ester Vázquez, an expert in gels and materials at the University of Castilla-La Mancha, in Spain, says ‘this idea … opens a new avenue for the development of reactions under more sustainable conditions’. She explains that most organic gelators rely on hydrogen bonds, which would compromise the stability of organolithium reagents. ‘To avoid this problem, the authors use hexatriacontane, a long-chain alkane that forms networks of platelet-like lamellar aggregates,’ she explains. ‘Clearly, these organic gels could encapsulate other hazardous and reactive species.’ Smith is collaborating with other chemists to study the viability of formulating the gels with tert-butyl lithium, a pyrophoric organometallic compound. Although they suspect gels could significantly lower its extreme reactivity with air and moisture, the studies on stability and safety are still preliminary. The compound is notoriously difficult to work with and was implicated in the death of UCLA research assistant Sheri Sangji in 2008.
Both Hevia and Vázquez envision that these encapsulated organolithium compounds could find applications in non-specialist laboratories. ‘Undergraduate students could learn about organometallic chemistry using the gel capsules,’ says Hevia. ‘We really hope that this approach will make reactive organometallic systems more accessible,’ explains Smith. ‘We also imagine the potential to do organolithium reactions in “kits” … opening up the possibilities [of organolithium gels] beyond teaching labs.’
Smith and collaborators are already exploring the commercialisation of the organolithium gels with industrial partners. ‘The gels could [arrive] in foil-wrapped single-use tablets … or provided in extrudable forms that replace the sure-seal capped bottles,’ he says. Both solutions could become ideal and useful ‘for high-throughput chemistry and discovery science’.
References
P Slavík et al, Nat. Chem., 2023, DOI: 10.1038/s41557-023-01136-x














No comments yet