Harnessing carbene reactivity with light
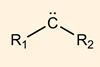
Two-step photocatalytic strategy produces metal carbenes easily and safely
Scientists in the US have used visible light to turn organic radicals into iron carbenes – attractive reactive intermediates with important applications in synthetic chemistry.1 The new approach, developed by a team working with Nobel Laureate David MacMillan, overcomes safety concerns about carbene synthesis, making these compounds easier to handle in the lab.
Metal carbenes are of interest to chemists because they can perform chemistry that is nearly impossible to achieve in other ways, says Nathan Dow from Princeton University in the US who participated in the study. ‘To many of us, cyclopropanation and bond-insertion reactions are practically synonymous with carbene chemistry – and the molecules that are made with these tools see use everywhere in science, especially in medicine.’