Highly reactive carbocations tamed in bond forming reaction
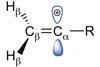
Chemists harness vinyl cations for hydrocarbon synthesis and discover an unusual mechanism along the way
Vinyl cations – highly reactive intermediates long thought to be too uncontrollable for synthesis – have been harnessed in carbon–carbon bond forming reactions. The researchers behind the work think that this strategy could allow them to transform biomass ketones into fuels or construct complex natural products.