A team in France has detected hydrogen dimers – one of the simplest molecular complexes – at room temperature for the first time.1
Researchers first recorded direct spectroscopic evidence of van der Waals hydrogen dimers, (H2)2, in the infrared spectrum of molecular hydrogen at 20K in 1964.2 The dimers were then detected in the atmospheres of Jupiter and Saturn in the 1980s as part of the Voyager IRIS mission.3,4 Until now, however, hydrogen dimers have only been studied at temperatures up to 77K due to their remarkably small bond dissociation energy of 3cm-1 and correspondingly low concentrations at elevated temperatures.
Now, Alain Campargue, Hélène Fleurbaey and Samir Kassi from the Grenoble Alpes University have used cavity ring-down spectroscopy (CRDS) to study hydrogen dimers at room temperature.1 CRDS is a high resolution spectroscopic technique that uses high-reflectivity mirrors to create a sample path length on the order of kilometres for ultrahigh sensitivity detection. Here, CRDS enabled the researchers to study hydrogen dimers at room temperature and at a calculated relative concentration of only 3.7ppm. ‘We were measuring H2 monomer lines in a metrological way to fulfil the needs of databases and challenge theoretical calculations,’ explains Fleurbaey. ‘[Then] we observed a series of unexpected features which motivated this study. This is a demonstration that one should never exclude phenomena supposing they are weak,’ adds Kassi.
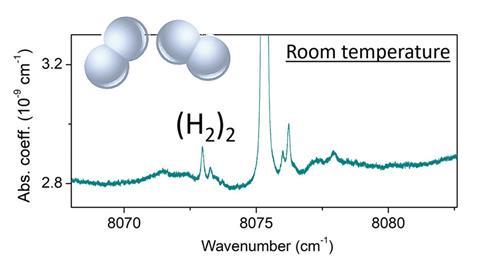
‘[This study is] really at the frontier between different fields, from chemistry to planetology and fundamental physics’, comments Clément Lauzin, a spectroscopy expert from the Catholic University of Louvain in Belgium. ‘The researchers have a smoking gun of evidence for these species. You can really see some features which are coming from the complex and not just from the short-lived species associated with collisions.’
The Grenoble team also calculated an average intermolecular distance of 4.5Å, which was in good agreement with the previously proposed range of 4.2–4.6Å.2 ‘For me, the most amazing result is the similarity between [the previous] spectra at 20K and their spectra at room temperature,’ adds Arnaud Cuisset, an expert in rovibrational spectroscopy from the University of the Littoral Opal Coast in France. This similarity occurs because, at both temperatures, only the lowest-lying rotational states of the vibrational ground state of the dimer are bound and result in sharp features in the spectra.The researchers would now like to measure the effect of temperature on these dimers which, so far, has only been theoretically predicted.
References
1 H Fleurbaey, S Kassi and A Campargue, Phys. Chem. Chem. Phys., 2024, 26, 21974 (DOI: 10.1039/d4cp02605e)
2 A Watanabe and HL Welsh, Phys. Rev. Lett., 1964, 13, 810 (DOI: 10.1103/PhysRevLett.13.810)
3 L Frommhold, R Samuelson and G Birnbaum, Astrophys. J., Lett., 1984, 283, L79 (DOI: 10.1086/184338)
4 ARW McKellar, Can. J. Phys., 1984, 62, 760 (DOI: 10.1139/p84-104)













No comments yet