A molecule that aggregates in bacteria and destroys them from the inside could offer a new way to treat infections while minimising the risk of antibacterial drug resistance.
The diarginine peptidomimetic molecule used to create this effect is essentially a small protein fragment containing two arginine amino acid units separated by a spacer group. The compound can enter bacterial cells, bind to bacterial DNA and subsequently rupture the bacterial cell membranes.
‘Once the compound enters a bacterial cell, it rapidly binds to and aggregates around the bacterial genomic DNA. This intracellular aggregation disrupts the normal spatial organisation within the cell, which is crucial for bacterial survival and other traits, such as resistance development,’ lead researcher Xinxin Feng, who is based at Hunan University in China, tells Chemistry World.
Although there is recognition that new antibiotic drugs are desperately needed, molecules that target bacteria in completely new ways are unusual. ‘This recent paper has an antibiotic mechanism that I have never seen before, and those are always welcome,’ wrote Derek Lowe, a chemist based at the Novartis Institutes for BioMedical Research, in a recent blog about the research. According to Lowe, ‘having an out-there mode of action is not necessarily a disadvantage’ for potential antibiotics due to the resistance many types of bacteria have developed towards ‘attacks through the normal channels’.
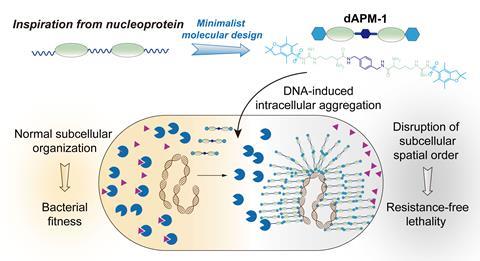
The diarginine peptidomimetic was initially developed in the lab and was shown to bind to double-stranded bacterial DNA. Feng and her colleagues tested it on so-called Eskape bacteria that are known to be susceptible to drug resistance, such as Staphylococcus aureus, and assessed whether it was toxic to eukaryotic cells.
The molecule proved most effective against gram positive bacteria in the lab and even showed good efficacy against methicillin-resistant S. aureus (MRSA). Following this, the researchers tested the diarginine peptidomimetic in rodents with superficial wounds, abscesses, and septicemia-like infections. The wound healing rate was significantly faster in animals treated with the candidate therapy. In addition, in the septicemia model where mice were infected with S. aureus bacteria, mortality improved from 0 to 50% after treatment.
Importantly, the diarginine peptidomimetic did not appear to be toxic to non-bacterial, eukaryotic cells. ‘The compound cannot effectively accumulate in eukaryotic cells due to its very low binding affinity for major eukaryotic cell membrane lipids, such as phosphatidylcholine and cholesterol,’ explains Feng.
Although these initial results are promising, this research is at an early stage and several hurdles will need to be overcome before it can reach the clinic. ‘The immediate next steps involve a thorough investigation of its mechanism of action, along with structural optimisation to enhance its in vivo antimicrobial efficacy,’ says Feng.
References
A Yang et al, J. Am. Chem. Soc., 2024, DOI: 10.1021/jacs.4c04749













No comments yet