Cryo-electron microscopy (cryo-EM) structures of a human taste receptor have revealed new information about the mechanisms that enable us to perceive bitter flavours.
Our ability to taste bitter flavours is the result of complex interactions between over 1000 compounds and a suite of 26 receptors, called type-2 taste receptors (TAS2Rs). However, understanding of these interactions has been hindered by a lack of structural data.
Now, researchers in the US and China have reported two cryo-EM structures of the human bitter taste receptor, TAS2R14. TAS2R14 spans across the membranes of cells on the tongue as well as numerous other human tissues and can recognise, and bind to, more than 100 structurally diverse tastant compounds that are associated with bitter flavours.
The new structures reveal the presence of two binding sites on the receptor: an allosteric site located inside the cell and an orthosteric site located on the extracellular part of the receptor. A tunnel, surrounded by hydrophobic amino-acid residues, appears to run between the two sites.
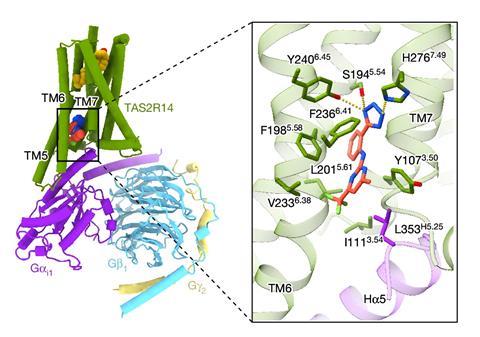
The researchers observed that the orthosteric site was occupied by cholesterol – a compound found in all animal cell membranes. The team believe that this puts the receptor in a semi-active state allowing it to be easily activated by the bitter tastant. It is thought that bile acids, which have a similar chemical structure to cholesterol, could also bind to, and activate, TAS2R14.
The only other TAS2R structure to have previously been determined is that of TAS2R46 and comparison with TAS2R14 reveals distinct structural differences, suggesting that each TASR2 receptor could bind to particular types of molecule.
During the project, the researchers discovered that the TAS2R14 protein is expressed in many other tissue types and is found in concentrations 100 times higher in the cerebellum than on the tongue. Further work is planned to uncover the role that the receptor plays in cellular signalling outside of the mouth.
References
Y Kim et al, Nature, 2024, DOI: 10.1038/s41586-024-07253-y

















No comments yet