A palladium-based cage that traps and twists long arylalkyne chains is behind a new method for synthesising polycyclic systems. Researchers from the University of Tokyo in Japan used the cage to induce a tetradehydro-Diels–Alder reaction, under mild conditions and high levels of selectivity.
Arylalkynes, like the ones used in this project, are conventionally used in a tetra-dehydro-Diels–Alder reaction to form polyclic systems. Traditional tetradehydro-Diels–Alder reactions tend to require temperatures exceeding 150°C. Such harsh reaction conditions and a lack of stereoselectivity in the product have seen chemists question how they can improve the reaction. The answer, it seems, is a molecular cage.
‘Molecular cages are in a sense biomimetic as they resemble enzymes, they’re water soluble and have hydrophobic cavities within them,’ says Georgi Genov, who worked on the study alongside Hiroki Takezawa, Harumi Hayakawa and Makoto Fujita. ‘Whilst these cages aren’t at all as functionalised as proteins are, they can share a lot of common features which is why they are useful for selective reactions like this.’ In this work the molecular cage is based on a Pd6L4 design that contains six palladium atoms at the corners of the cage. Encompassing four sides of the cage are triazine-type compounds, acting as ligands.
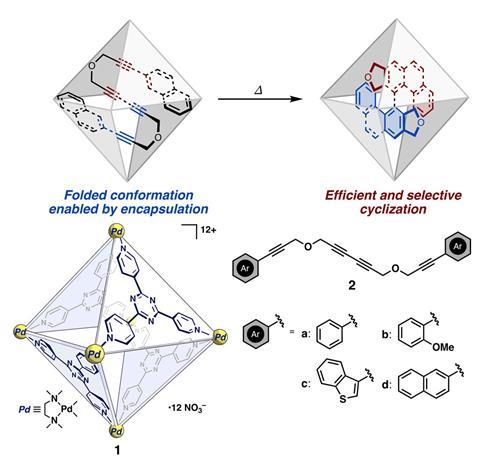
The reaction proceeds by adding the molecular cage, which is solvated in deuterated water, to the arylalkyne. Encapsulating the arylalkyne in the palladium cage forces it to twist and contort into a configuration where the reaction sites are near one another. The close proximity of the alkyne functional groups initiates a cyclisation reaction. Removing the now cyclised product is easy, a simple organic work-up frees it from the cage. The reaction proceeds at around 80°C in an aqueous solution meaning it is milder than other cyclisation options for the molecule.
Takezawa says that this work is ‘a great example of how the type of cavity in the molecular cage, can control the shape of the product’. The group has used this cage before to fold a set of aromatic amides, bringing their reaction sites together. These techniques Genov says, ‘help to overcome the entropic barrier that can be associated with the reaction’. The conformation the cage induces in the arylalkyne means that the triple-bonds are aligned in such a way that only one polycyclic conformation is available as a product. This is in contrast to previous tetradehydro-Diels–Alder reactions that struggle to control the selectivity.
Experts in the field of supramolecular chemistry are impressed with the work. Angela Grommet from Chalmers University of Technology in Sweden, says her first reaction to seeing the results was ‘Wow, the Fujita group has done it again.’ She goes on to say that it is ‘a really nice example of the principle of geometrically constricting a molecule in order to achieve changes in selectivity’.
Imogen Riddell from the University of Manchester, UK, calls the work elegant. It is ‘one of the best performing cages that supramolecular chemists have come up with.’ She is hopeful the work will add fundamental knowledge to the area and ‘let chemists move forward towards cleaner, greener and more efficient reactions’.
The Fujita group sees their work as a stepping stone to even more examples of confined reactions. ‘We want to look at different shaped cage to see if we can obtain a different isomer,’ says Genov, ‘the future will hopefully mean that we can design the right cage for the right product.’ And Takezawa wants to extend the field of substrates: ‘I think the next target should be looking at a sterically hindered molecule and then maybe if we used a cage with a smaller cavity, we could help to synthesise an even more angular or distorted organic compound.’
References
G R Genov et al, J. Am. Chem. Soc., 2023, DOI: 10.1021/jacs.3c06301












No comments yet