Molecular decoration determines origin of MOF acidity
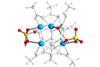
Analytical combo used to pinpoint strong Brønsted acid site in promising next-generation solid acid catalyst
Researchers have pinpointed the strong acid site of a sulfated-zirconium MOF catalyst by identifying a specific molecular decoration within its structure. The work could aid studies into solid acid-catalysed reaction mechanisms as well as the development of next-generation solid acid catalysts for many industrial applications.