Molecular energy transfer put under the microscope
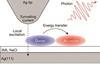
Combining scanning tunnelling microscopy with luminescence gives access to energy transfer in action

Combining scanning tunnelling microscopy with luminescence gives access to energy transfer in action