Multitasking boron catalyses first aldol reaction from esters
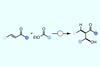
Using esters instead of aldehydes offers chemists a completely new retrosynthetic disconnection
The first aldol reaction that uses esters instead of aldehydes as one coupling partner has been developed by scientists in the UK. It will give chemists ‘an entirely new retrosynthetic disconnection,’ says the study’s leader Stephen Thomas from the University of Edinburgh.