Nanoparticles poison single-atom cross coupling catalyst
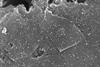
In a classic heterogeneous palladium catalyst, less than 1% of metal does 99% of catalytic work
Tracking a palladium catalyst in space and time has revealed that nanoparticles are a catalyst poison: they capture and inactivate the small amount of single atoms that do the overwhelming amount of catalytic work.
‘This is very surprising outcome and contradicts the generally accepted approach to catalyst design in this area,’ says Valentine Ananikov from the Russian Academy of Sciences. ‘In many studies of catalytic cross-coupling reactions, as well as many other reactions in fine organic synthesis, it is typically assumed that metal nanoparticles represent an active form of the catalyst.’
