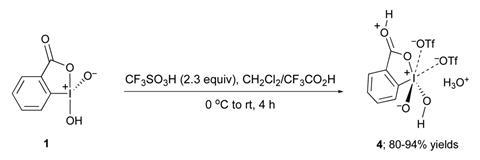
2-iodoxybenzoic acid ditriflate can oxidise stubborn hydrocarbons and obstinate polyfluoroalcohols
Scientists have made 2-iodoxybenzoic acid ditriflate – the most powerful hypervalent iodine(v) oxidant to date. The new compound can directly oxidise polyfluorinated alcohols, substrates so resistant to oxidation that synthetic chemists use them as solvents with other hypervalent iodine reagents.
Iodine compounds regularly serve as sustainable alternatives to metals in organic synthesis. Hypervalent iodine reagents in particular have very useful oxidising properties but suffer from underuse in both academia and industry. Increasing the range of compounds that hypervalent iodine reagents can oxidise should help them emerge from obscurity. ‘Hypervalent iodine reagents will likely become even more popular with the increasing importance of green chemistry due to their low toxicity,’ says Berit Olofsson, an expert on hypervalent iodine from Stockholm University, Sweden, who was not involved in the research.
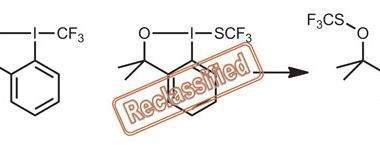
The oxidant prepared in this study is 2-iodoxybenzoic acid-ditriflate (IBX-triflate). ‘Any chemist who needs to oxidise a particularly resistant organic or inorganic compound should be interested in this new reagent,’ comments Viktor Zhdankin from the University of Minnesota, US, who contributed to the discovery. Preparing IBX-triflate involves treating 2-iodoxybenzoic acid with two molecules of trifluoromethanesulfonic acid in trifluoroacetic acid to form a thermally stable, crystalline precipitate. This precipitate is IBX-triflate. ‘It is surprisingly easy to make this compound considering how powerful of an oxidant it is,’ says Alexandr Shafir, who investigates hypervalent iodine in oxidative C–C bond formation at the Institute of Advanced Chemistry of Catalonia (IQAC-CSIC).
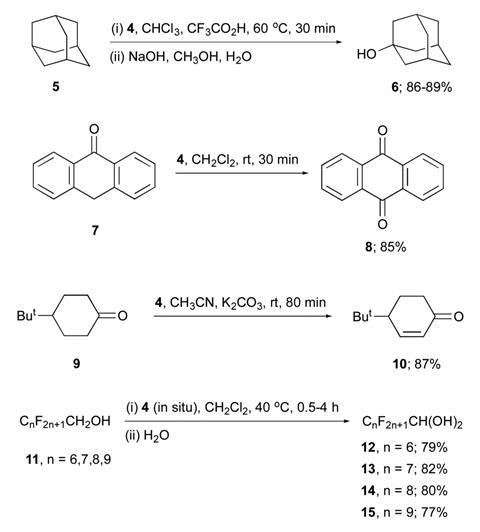
Previously, organic derivatives of pentavalent iodine have been the most powerful. However, these cannot oxidise polyfluorinated alcohols. ‘We have demonstrated that IBX-ditriflate readily oxidises numerous primary polyfluorinated alcohols to the corresponding perfluorinated aldehydes,’ explains Zhdankin. This is possible due to the presence of the non-nucleophilic triflate anion giving the IBX-ditriflate iodine atom a significant positive charge and dictates its extraordinary electrophilic and oxidising reactivity.
‘IBX-triflate readily oxidises almost any organic matter, which makes it difficult to handle. It oxidises even the most stable solvents,’ continues Zhdankin. ‘To make such a powerful oxidant comes at a price. If you want to work in solution, you need to have a solvent that withstands this species. This is what these researchers figured out,’ adds Shafir. ‘Right now I cannot imagine all of the applications that may come from this.’
References
This article is free to access until 3 August 2019
M S Yusubov et al, Chem. Commun., 2019, DOI: 10.1039/c9cc04203b







1 Reader's comment