New kind of heterocycle made in just one easy step
Simple synthesis makes once inaccessible class of fused seven-membered ring system
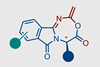
Chemists in Austria have discovered a never-before-seen class of heterocycle and made it in a single reaction.
The team around Nuno Maulide from the University of Vienna was surprised to find an unusual product after combining α‐phthalimido‐amides with triflic anhydride – a strong electrophile – and a base in acetonitrile as the solvent. Although the reaction has similar ingredients to the electrophilic amide activation, it produces an entirely different product, incorporating the solvent to create a new type of heterocycle. The fused, seven-membered rings contain one oxygen and two nitrogen atoms.
