What began as an effort to improve an established reaction has led to the discovery of an entirely new pathway in chemistry.

Christian Hering-Junghans and Torsten Beweries, leading a team at the Leibniz Institute for Catalysis, have overturned long-held assumptions about the Wittig reaction – a process that has been fundamental to chemistry for nearly a century. This breakthrough also introduces a new way to create triazabutadienes, a class of molecules with applications in synthesis, biochemistry and polymer chemistry.
‘Triazabutadienes are currently made by combining organic azides with N-heterocyclic carbenes. However, this will always lead to the carbonyl carbon atom being incorporated in an N-heterocycle,’ says Hering-Junghans. This limits the types of triazabutadienes that can be synthesised. ‘Our methodology basically allows us to explore new substitution patterns,’ he adds.
This new approach has garnered attention for its potential. Michael Cowley at the University of Edinburgh, who was not involved in the study, sees it as a ‘quite straightforward way to prepare diverse triazabutadienes’, which could be used for tagging or functionalising proteins.
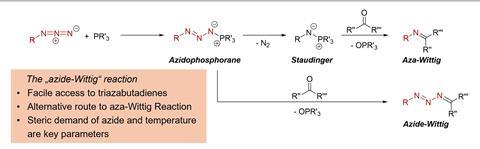
The Wittig reaction is one of the go-to methods for creating carbon–carbon double bonds with precise control over the reaction outcome. However, when the team initially tried to develop new phosphorus ligands using the phospha-Wittig reaction, something unexpected happened: only one aldehyde group in a dialdehyde was converted into the ‘phosphaalkene’ unit (Mes*P=C(H)R).
This surprised the team and led them down an entirely new path. Hering-Junghans says he saw the free aldehyde group as an opportunity to insert a nitrogen–carbon double bond in addition to the phosphorus–carbon double bond.
‘This was the first time we had a precursor with both a phosphaalkene and an aldehyde unit present at the same time,’ says Hering-Junghans. ‘As conversion into a second phosphaalkene unit could not be achieved, the aza-Wittig reaction seemed the obvious choice to install an imine unit next to [it].’
Beweries elaborates, ‘These types of ligands are well-established for various types of transformations in organometallic chemistry and homogeneous catalysis. But this reaction did not succeed [as expected].’
NMR spectroscopy indicated that the nitrogen–carbon double bond had been incorporated, but the data was ambiguous. ‘X-ray crystallography was key to understanding what had happened,’ says Beweries.
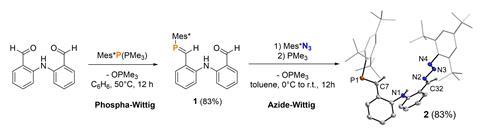
Instead of incorporating just one nitrogen atom into the ligand, the entire azide unit had been transferred into the product, yielding a triazabutadiene, rather than the expected imine. Normally, the formation of an imine would release nitrogen, as this is typically the most energetically favourable pathway. However, the bulky groups already present in the ligand prevented this, stabilising the nitrogen and keeping it bound to the molecule. This led to a new reaction pathway, which they dubbed the Azide-Wittig reaction.
‘It’s always exciting to see an old dog do new tricks,’ comments Laura Cunningham at the University of Galway who was not involved in the study. ‘The Aza-Wittig reaction has been around for decades, so this discovery serves as a reminder that there can be something new right under our noses.’
Cunningham also points out that being able to convert aldehydes into triazabutadienes in one step means their synthetic versatility is significantly increased. ‘The formation of triazabutadienes from something as ubiquitous and diverse as aldehydes might lead to far greater interest and new applications.’
While there may be limitations to the methodology that still need to be overcome, Cowley emphasises the importance of flexibility and embracing unexpected outcomes. ‘This result didn’t arise from an attempt to solve a specific problem, but it still uncovered useful new reaction pathways and possibilities,’ he notes. ‘The main takeaway from the paper is: “this transformation is possible”. From my perspective, as someone who approaches chemistry with a focus on “What can we make?” rather than “How can we make it?”, potential limitations in the transformation don’t reduce the value of knowing it’s possible in the first place.’
References
Kushik et al, Angew. Chem., Int. Ed., 2024, DOI: 10.1002/anie.202412982











No comments yet