Azobenzene compounds can reversibly switch between crystalline and amorphous states to capture gases
A new set of compounds that can be reversibly switched between crystalline and amorphous isomers by light has been developed by researchers in Italy. In its crystalline state, the material is highly porous and has an almost unprecedented selectivity for carbon dioxide, so the researchers say it could find use in carbon capture technologies.
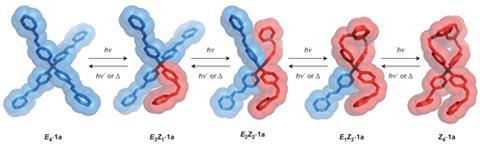
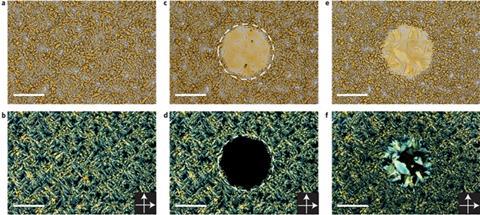
Azobenzene reversibly switches between a linear E isomer and a buckled Z isomer when irradiated by ultraviolet light. However, spatial crowding usually inhibits the shape change in the crystalline state. Now, Alberto Credi at the University of Bologna and colleagues have synthesised a series of tetra(azobenzene)methane compounds comprising four E-azobenzene units covalently bonded to a tetrahedral carbon atom. The central carbon atom keeps the E-azobenzene arms apart, producing a very low density crystal which can absorb other small molecules. The interconnected pores in the crystal are able to trap carbon dioxide from nitrogen mixtures with a selectivity ratio up to 80:1, making it a promising candidate to filter carbon dioxide out of the exhaust gases of cars or industrial chimneys.
On irradiation by near-ultraviolet light, the azobenzene units transform into the Z isomer, disrupting the crystal structure. The material becomes a viscous liquid, releasing the trapped gas. On irradiation by visible light or heating to 130ºC for 10 minutes, however, the azobenzene recrystallises, allowing the material to absorb carbon dioxide again. ‘One can, in principle, think of a device that can be loaded with carbon dioxide in the dark,’ says Credi. ‘Then you open a window or make a screen become transparent: the radiation will squeeze the sponge and release the carbon dioxide.’ The team is now investigating other molecules based on the same architecture and looking at further possible applications.
Other researchers are impressed by the potential of the materials. ‘I think this is a beautiful example of how one can control – by molecular design – important properties of solid materials,’ says Ben Feringa of the University of Groningen in the Netherlands. ‘I think these kinds of responsive materials have a lot of potential for separation technology but maybe also for several other things like catalysis, changing electronic properties.’
Christopher Barrett of McGill University in Canada adds a note of caution: ‘They have modest porosity changes,’ he says. ‘I’m not going to buy any stock yet in the applications.’ Nevertheless, he insists: ‘It’s a new scientific discovery, and it means that somebody can take this discovery and improve it.’












No comments yet