One-electron covalent bond between two carbons pushes limits of bonding
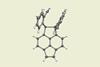
Linus Pauling proposed exotic bonds but they have never been seen between two carbons until now
Scientists in Japan have made a groundbreaking discovery: they have identified a covalent bond between two carbon atoms that share only a single electron. ‘This [study was driven] by a curiosity to know where the limits of chemical bonds are,’ says Takuya Shimajiri at the University of Tokyo.
One-electron bonds were first proposed in 1931 by Linus Pauling and have since been reported between heteroatoms. However, no direct evidence of their existence between carbon atoms had ever been observed. ‘We came up with the idea that it would be possible under the proper molecular design,’ says Shimajiri.
