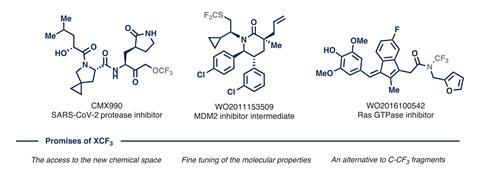
Chemists at the University of Amsterdam have developed a method that avoids the use of per- and polyfluoroalkyl substances (PFAS) in the synthesis of fluorinated compounds. Their approach offers a more environmentally-friendly route to compounds with a trifluoromethyl group attached at a sulfur, nitrogen or oxygen, which provides unique features for drug molecules and agrochemicals.1

Many pharmaceuticals, such as anti-depressants, and agrochemical compounds like pesticides include at least one trifluoromethyl group to enhance hydrophobicity and increase metabolic stability, which improves efficacy and lowers the effective dose required. Bespoke fluorinated reagents are typically needed to introduce these CF3 groups, but many PFAS compounds are facing legislation in the US, Europe and other parts of the world because they have been linked to health problems such as decreased fertility, developmental effects and cancers. But the University of Amsterdam team, which includes scientists from drug giant AstraZeneca, says its new synthesis protocol offers an alternative because it only requires a caesium fluoride salt – which is not a PFAS – as the fluorine source.
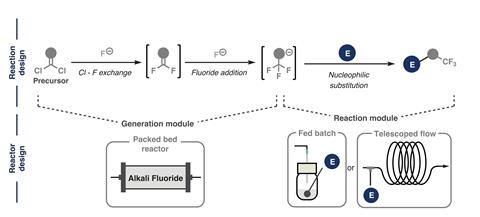
The microfluidic platform passes chlorocarbon chemicals through a packed bed flow reactor containing the caesium fluoride salt. The reactor brings the two reagents into close contact resulting in efficient fluorination of the chlorocarbons, producing trifluoromethylated anions. These anions can then be reacted with drug or agrochemical precursors to make the trifluoromethylated molecules of interest.
Ian Cousins, an environmental chemist at Stockholm University in Sweden, says that the X–CF3 chemistry, where X is S, N or O, is ‘an interesting building block’ that ‘still falls under the broad definition of PFAS’. However, he suggests that if these substances are shown to be fully degradable or mineralisable they could be excluded from forthcoming European regulations governing PFAS.
A recent study2, which was part of a larger project that Cousins coordinated called Perforc3 – a Europe-wide doctoral training programme in the field of PFAS contaminants – also looked at a similar approach to this one. But Cousins notes that it found that these prototype substances containing trifluoromethoxy groups also led to ‘dead-end persistent metabolites’, and therefore he argues that one cannot conclude that they are all degradable and recommends that such substances be tested on a case-by-case basis.
References
1 M Spennacchio et al, Science, 2024, DOI: 10.1126/science.adq2954
2 V Licul-Kucera et al, Chemosphere, 2024, DOI: 10.1016/j.chemosphere.2024.141237
















No comments yet