A new photochemical reaction can create glycosidic bonds in ‘naked’, native sugars, without the need of protecting groups.
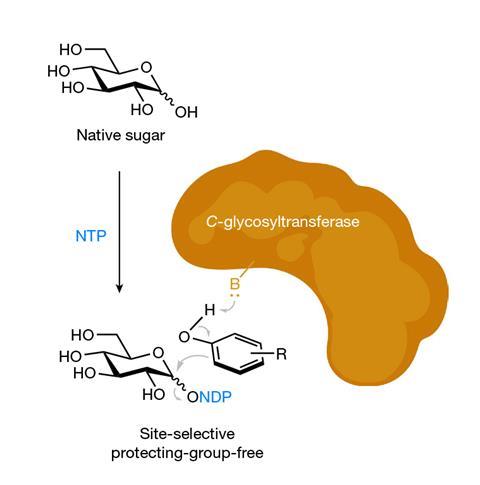
Whereas state-of-the-art synthetic methods use several steps, researchers have developed a ‘reactive intermediate’ that’s stable under bench conditions and easily couples with a variety of nucleophiles, including proteins. ‘I would have benefitted directly from this approach during my graduate work,’ says 2022 Nobel laureate Carolyn Bertozzi, an expert on sugar chemistry at Stanford University in the US. Replacing the oxygen in glycosidic bonds by other atoms, like carbon in the case of C–glycosides, makes more stable sugars and sugar mimics, with applications in drug development.
Here, researchers functionalise sugars directly and efficiently, giving access to useful bioactive molecules, resistant to enzymatic degradation thanks to the anomeric carbon–carbon bond, explains Bertozzi. ‘Back in the day, radical C–glycosylation reactions required many more steps and the use of toxic metal initiators – not fun!’ she says. Even the best approaches, which worked on native sugars too, take four or five steps, sometimes with harsh reagents and generating a lot of waste. ‘The use of a photoredox radical mechanism was a clever choice, as the chemistry is mild, selective and compatible with aqueous solvents, which are required with free sugar substrates,’ adds Bertozzi.
‘This method directly transforms native sugars – the most abundant form in nature – into various classes of glycosyl compounds and glycoproteins,’ explains Ming Joo Koh, from the National University of Singapore, who co-led the study. ‘We provide a robust platform to employ native sugars directly as substrates.’
The method is inspired by enzymatic glycosylation reactions, where the anomeric position is activated and then substituted by a substrate, in a site-selective and stereocontrolled manner. In this case, researchers replace the anomeric hydroxyl group with an electron-withdrawing thiol. This intermediate is easy to isolate and sufficiently stable to survive on the bench for months without any degradation or decomposition. Yet, a little light sparks its reactivity. ‘The thioglycoside [intermediate] is sufficiently redox-active to undergo a stereocontrolled, photoinduced glycosylation in the presence of blue light, a reductant and a base,’ says Koh.
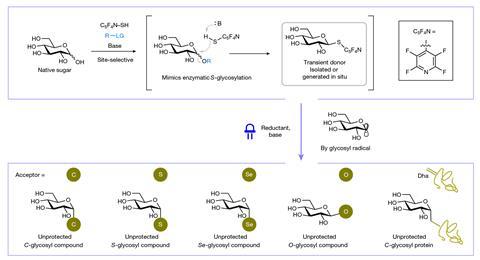
‘It’s a very exciting development in the field, particularly because the process is simple and very efficient with off-the-shelf reagents,’ explains Carmen Galán, an expert in sugar chemistry at the University of Bristol, UK. ‘Despite many advancements in the field, the stereoselective chemical synthesis of glycosides remains a formidable challenge,’ she says.
To yield similar results, most glycosylation reactions ‘rely on the introduction of a latent leaving group at the anomeric position, activated in the presence of a nucleophile to undergo the coupling reaction’, explains Galán. Moreover, often other protecting groups provide regio- and stereoselectivity, always strategically selected for each sugar, she explains. The complexity of protection, deprotection and all the additional steps – and probably purification processes, too – creates a huge bottleneck in the synthesis of glycoside compounds. In this paper, the authors overcome most hurdles. ‘The method obviates the use of protecting groups in the glycosyl donor by activating the anomeric alcohol with a “capping reaction” that generates a reactive intermediate,’ continues Galán. The intermediate undergoes a photoinduced reaction and creates glycosidic bonds with carbon, sulfur, selenium and oxygen. All these ‘high value’ chemical compounds come from a ‘very clean’ process, she says. ‘The conditions are mild, allowing for a better control and fewer side products … very reminiscent of natural enzymatic processes.’
‘Nature’s doing the same thing,’ according to Ben Davis from the University of Oxford, UK, who co-led the study. ‘It’s remarkable , we are full of UDP–glucose, which is a stable [yet] potentially highly reactive sugar, pulsing through our bodies all the time,’ says Davis. ‘But nothing happens to it until it reaches the right space.’ Something similar happens to the thioglycoside intermediate, stable and safe until the light is turned on. ‘We found another sweet spot to store latent reactivity in an unprotected [sugar] molecule, ready to go.’
The versatility of the glycosylation extends to the direct functionalisation of proteins, which until now was also quite complicated for chemists. ‘I am impressed with radical C-glycosylation on … proteins under biocompatible conditions,’ says Bertozzi. ‘This adds to the toolbox of protein modification methods for biotechnology applications,’ she says. Because of the long-term stability of the ‘capped’ thioglycoside intermediate, the concept could be scaled up and commercialised. ‘After optimisation, one could envision using flow photochemical reactors to carry out some of the reported transformations,’ adds Galán.
References
Y Jiang et al, Nature, 2024, DOI: 10.1038/s41586-024-07548-0















No comments yet