Plutonium to carbon double bond a first
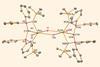
Findings offer insight into differences in chemical reactivity between actinides and lathanides
Almost 60 years after the first organo–plutonium complex was reported, researchers have prepared the first complex with a plutonium carbon (Pu═C) double bond. They say the finding provides the opportunity for chemists to understand more about the divergence in properties and chemical reactivity between actinides and lanthanides.
The first organo-plutonium complex (Pu(C5H5)3) was first reported in 1965 but research into the fundamental properties of plutonium has been held back due to experimental difficulties and availability of the element. ‘Uranium is probably the last element in the periodic table where you can work in a normal laboratory,’ explains Steve Liddle, head of inorganic chemistry at the University of Manchester and one of the researchers on the study. ‘Go one place to the right and you need a completely different radiological lab setup.’
