Polar solvents promote halogen bonds over hydrogen ones
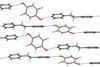
Solvent decides bonding battle winner in supramolecular systems
Solvent effects control competition between hydrogen bonding and halogen bonding in supramolecular systems, new research shows. The upshot of the finding is a potential new tool to direct supramolecular self-assembly.