Polycyclic hydrocarbons finally make waves
New aromaticity insights could help advance organic electronics
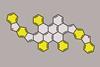
Scientists based in Germany have synthesised two polycyclic hydrocarbons with 64 π-electrons and an unusual undulating shape. The compounds’ aromaticity can be tuned by altering the direction of the cyclopenta[b]fluorene components in the molecules.