Pushing praseodymium past its oxidation limits
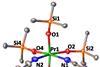
Researchers isolate the first molecular species bearing praseodymium(IV), which could find applications in catalysis and new materials
A team of chemists led by Marinella Mazzanti from EPFL, Switzerland, has managed to isolate a complex of praseodymium(IV) for the first time.1
Normally, lanthanide ions are at their most stable in their +3 oxidation state. Further oxidised +4 species are hard to find, particularly those that form molecular complexes. Until last year, chemists had only identified one lanthanide that could form stable molecular complexes in its +4 oxidation state – cerium. Its strong oxidising power rapidly led to myriad uses in catalysis, materials science and the separation of rare earths. Attracted by cerium’s versatility, researchers have been trying to find other highly oxidised lanthanide ions.
