Quantum chemical analysis uncovers previously overlooked contributor to carbocation stability trend
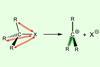
Introducing substituents destabilises the parent substrates
It is easier to form more substituted carbocations because of destabilisation in the parent substrate, rather than stabilisation in the reactive intermediate, new research shows.