Rare red californium sandwich pushes frontier of isolable molecules
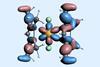
Researchers rehearsed metallocene’s synthesis with other elements before using just two milligrams of the precious radioactive actinide
Researchers from the US and China have made a rare californium sandwich compound. Working with a limited supply of the radioactive element, the group managed to obtain a crystal structure of a californium metallocene – revealing new insight into the chemistry of the heavy actinides.
