Retro-Diels–Alder study links solvent viscosity to reaction rate under microwave heating
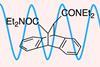
New study helps to establish the parameters for microwave-specific rate enhancements observed in certain reactions
Scientists in the US have unveiled a rate-enhancing effect of microwave irradiation on a high-temperature retro-Diels–Alder reaction. The group, led by Greg Dudley at West Virginia University, designed a reaction system that exploits a difference in polarity between the reactants and products. Following an extended debate over the existence and origin of microwave-specific effects in organic reactions, the group says their findings highlight the untapped potential of selective microwave heating in modern synthetic chemistry.