Despite its low cost and low toxicity, bismuth has found limited applications in organic synthesis. Liam Ball is working to change that
‘Bismuth has had its ups and downs over the decades – a bit like the economy,’ says Liam Ball. But while the global economy is floundering at best, organobismuth chemistry is very much on the up and Ball’s research exemplifies this progress.

Ball’s PhD was on gold-catalysed coupling methods, but he came to work with bismuth after a referee of one of his PhD manuscripts requested he synthesise a specific organogold compound – one for which the only literature method involved bismuth. Although Ball says that approach ‘ultimately failed in [his] hands’, it sparked his interest in the field. As he delved deeper, he began to identify opportunities to harness reactivity that had largely lain fallow since the late 1980s.
Organic chemistry with bismuth began in the mid-19th century with Carl Jacob Löwig and Eduard Schweizer’s synthesis of triethylbismuth. However, interest in the field remained limited for decades, as these compounds were often seen as unstable or less reactive than their lighter group 15 counterparts.
Ball explains that while Derek Barton pioneered the use of organobismuth(v) reagents in the 1980s, particularly in oxidative transformations, much of this work was later eclipsed by the rise of more efficient cross coupling methods like the Suzuki and Chan–Lam reactions. ‘But now I think we’re recognising the opportunities for using bismuth to address challenges that you can’t address with cross coupling.’
Bismuth stands out among heavy metals for its low toxicity and relatively low cost – factors that, according to Ball, helped spark renewed interest in the element in the late 20th century. ‘Most of the things you can do with organolead, you can do with organobismuth,’ but bismuth is obviously much more ‘palatable to work with’.
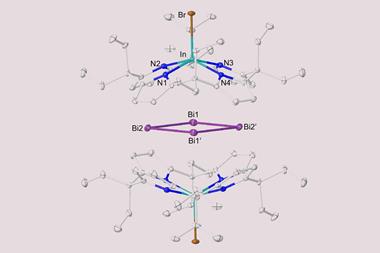
Broadly, Ball’s team is working to make the bismuth chemistry demonstrated by Barton more practical and easier to perform. Indeed, most of the bismuth(iii) and bismuth(v) compounds resulting from Ball’s lab are bench stable, ‘so you can handle them, weigh them out as you would any other stable solid’.
Bismuth-mediated chemistry
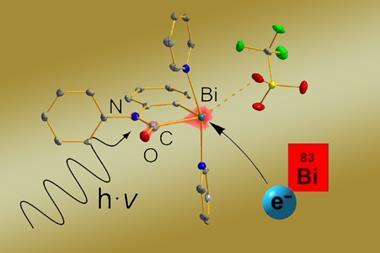
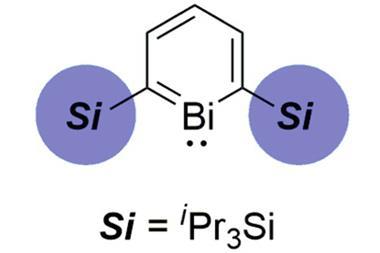
A key output from Ball’s lab was a general bismacycle reagent, synthesised via a one-pot boron-to-bismuth transmetallation from readily available boronic acids.1 This reagent, with bismuth in its +3 form, serves as a precursor to the more reactive bismuth(v) species. ‘The plus five oxidation state is where, certainly in terms of the products you form, the interesting stuff starts to happen. Bismuth(v) is oxidising, and – with a weak bismuth–carbon bond – it acts as an aryl electrophile, reactivity which is not necessarily trivial to access. And so you can add that onto various nucleophiles, particularly weak nucleophiles that you cannot engage in cross coupling very effectively.’
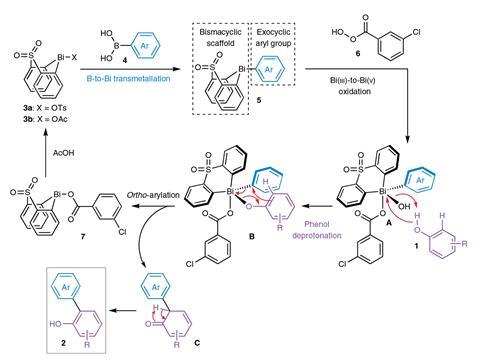
One transformation the team has used its reagent for is C–H functionalisation, including ortho- and meta-selective arylation of phenols.2 ‘C–H functionalisation obviously has the promise of reducing step count and improving atom efficiency.’
In collaboration with Syngenta, Ball and his team have also used their bismuth reagent to arylate cyclic diketones,3 creating the central motif in an emerging class of herbicides called ACCase inhibitors that is not only hard to make, but whose preparation tends to rely on lead-based reagents.
After realising the reagent was general and effective, they have since used it to O-arylate pyridones – a notable shift from the typical N-selectivity seen in such reactions.4 This chemoselectivity gives way to ‘complementary disconnections to the normal way of making aryloxypyridines, which are found in a lot of drugs and agrochemicals, and provides a way of accessing more diverse substrates.’
While each transformation requires tailored conditions, Ball was struck by how broadly applicable the reagent proved to be: ‘It seems to be a quite a privileged architecture in that regard.’ However, it’s not without its limitations, including needing to use stoichiometric quantities of the bismacycle. ‘You put a lot of mass in – bismuth is heavy – and you generate a lot of mass as waste, so that clearly has sustainability and cost implications,’ says Ball. He points to work by Josep Cornella’s group at the Max Planck Institute for Coal Research in Germany, which has demonstrated that catalytic methods using similar scaffolds are feasible. However, translating all stoichiometric methods into catalytic ones remains a significant challenge.
Earlier this year, Ball was named the chemistry laureate at the 2025 Blavatnik Awards for Young Scientists in the UK, in recognition of his contributions to the safe and sustainable synthesis of molecules vital to healthcare and agriculture. He emphasises that the honour reflects the collective effort of his team and the broader research infrastructure: ‘Research is not done by one person. It’s always a team, and not just the people in the lab, but the technicians who maintain the instruments, the admin staff who keep the university working, our collaborators, the sponsors, the funders. So, it’s a massive recognition of everyone who’s contributed to our research.’

Having recently marked its 10th anniversary, Ball’s research group is not only reviving interest in a once-overlooked element but also opening new avenues for synthetic chemistry. ‘I think there’s still a lot that chemists can do with bismuth,’ he says.
References
1 M Jurrat et al, Nat. Chem., 2020, 12, 260 (DOI: 10.1038/s41557-020-0425-4)
2 A Senior, K Ruffell and LT Ball, 2023, Nat. Chem., 15, 386 (DOI: 10.1038/s41557-022-01101-0)
3 K Ruffell et al, Angew. Chem. Int. Ed., 2022, 61, e202210840 (DOI: 10.1002/anie.202210840)
4 K Ruffell et al, Angew. Chem. Int. Ed., 2022, 61, e202212873 (DOI: 10.1002/anie.202212873)










No comments yet