System established by measuring how quickly electrophilic fluorinating reagents react with a common set of nucleophiles
Researchers from the UK have established a reactivity scale of commonly used electrophilic fluorinating reagents that chemists could use when planning a synthesis.
Many pharmaceutical molecules contain fluorine atoms, as these can improve the biological activity and metabolic stability of a drug. Natural organofluorine precursors are scarce and introducing a fluorine atom at a specific position is a key synthetic challenge. One selective method is electrophilic fluorination.
Electrophilic N–F fluorination occurs when a carbon nucleophile reacts with an electrophile bearing an N–F bond to form a new C–F bond. Such fluorinating reagents are widely used as they are easy to handle, but the choice of reagent typically results from trial and error.
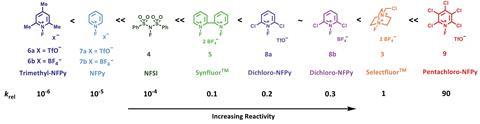
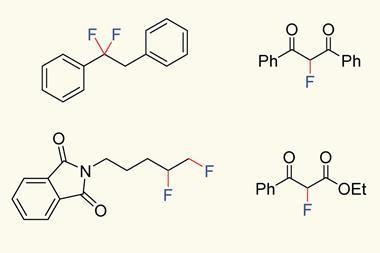
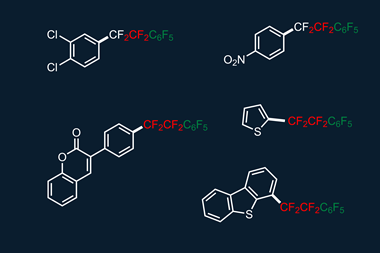
Now, David Hodgson from Durham University and his colleagues have determined a quantitative reactivity scale of reagents for performing N–F fluorination. By measuring how quickly 1,3-dicarbonyl model substrates were fluorinated using 10 commonly used fluorinating reagents, they devised a reactivity scale spanning eight orders of magnitude.
The team hope this scale will simplify the process of fluorination reagent selection for synthetic chemists.






1 Reader's comment