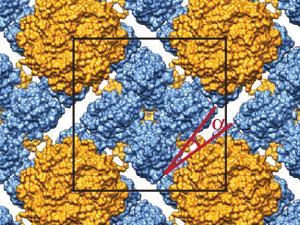
Graphene-containing microfluidic device used to study the structures of complex protein crystals
Introducing graphene into microfluidic devices can make it easier to study proteins at an atomic level, scientists in the US have shown. Devices that are thinner and interfere less with the measurements allow larger and more intricate protein structures to be resolved using techniques that rely on probing thousands of microcrystals.
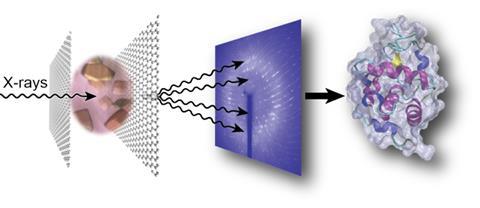
Understanding the structure of proteins on the atomic level is crucial, particularly when it comes to designing drugs. Traditional x-ray crystallography techniques rely on large crystals, which are difficult to grow for complex proteins and susceptible to radiation damage. More recently, serial crystallography using x-rays has become a leading method. This involves firing a beam of radiation from a synchrotron source is at thousands of tiny crystals – around 1–10µm in size – to form a cumulative overview of the protein’s structure.
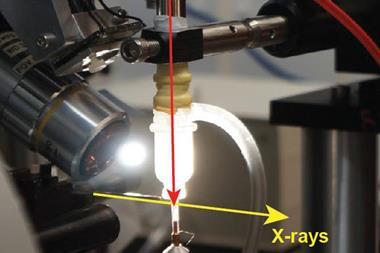
But sometimes transferring the microcrystals from their growth medium to the sample chamber can cause damage. To address this, microfluidic techniques are used to allow the crystals to be grown directly on-chip with minimal intervention. But doing this introduces fresh challenges. ‘The current microfluidic technology tends to be limited by the thickness of the device and the amount of noise that you would get when you try and do on-chip analysis,’ explains Sarah Perry from the University of Massachusetts Amherst in the US, whose group have addressed this issue by incorporating a large-area single-layer graphene film into the device.
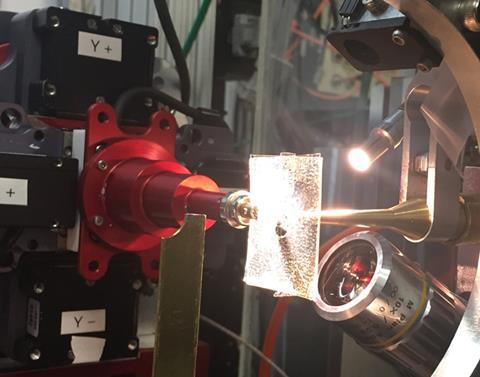
Not only does this reduce the device’s thickness, improving the signal-to-noise ratio, the graphene also acts as a barrier to prevent the sample evaporating. John Helliwell, an expert in crystallography at the University of Manchester in the UK, explains that preventing water loss from the crystal is ‘vital…because the sample hydration state needs to be preserved for its molecular integrity’.
Using hen egg white lysosome as a model, Perry and her group obtained an excellent signal to noise ratio in x-ray diffraction measurements of crystals grown and analysed within their device.
Helliwell describes the use of graphene as ‘a beautiful and totally elegant way’ of minimising background scattering, enabling analysis of ever more complex protein structures.
Perry’s group are now focusing on shrinking down the dimensions and increasing the complexity of the device, as well as studying the structure of proteins involved in programmed cell death.
References
S Perry et al,Lab Chip, 2016, DOI: 10.1039/C6LC00451B







No comments yet