Shape-shifting carbon ring helps researchers test alternative weed management tactic
Pausing plant growth, rather than completely inhibiting it, might make weeds less inclined to develop resistance
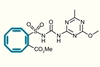
A compound designed and synthesised by researchers in Australia can stop an invasive plant known as rubber vine from growing for six weeks. The work sheds new light on the little-studied process of herbistasis and explores how researchers could remodel existing herbicides as herbistatic agents.