A photoredox-induced radical pathway is behind a synthetic strategy that allows users to insert a small ring into a heterocyclic parent ring. Frank Glorius from the University of Münster in Germany, who developed the method with Kendall Houk at the University of California, Los Angeles in the US, compared it to sleight-of-hand magic. ‘Maybe a magician throws two solid rings in the air or hides them under a cloth, but the next time the audience see them they’re connected. This is exactly what’s happening in our work, only it’s not a cheap trick, it’s catalytic activation.’
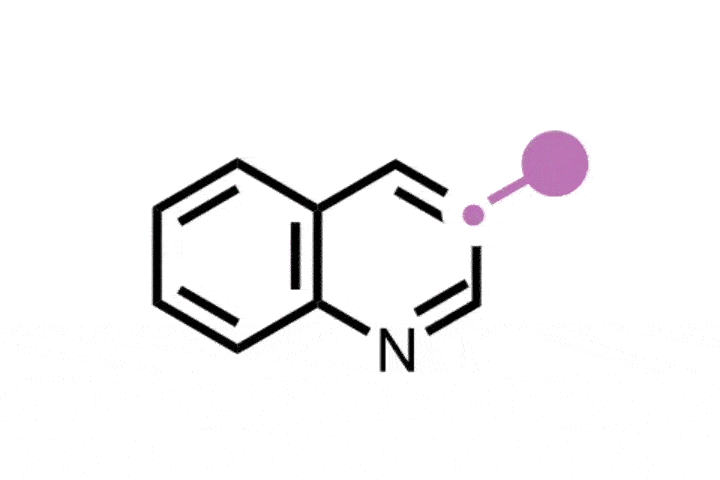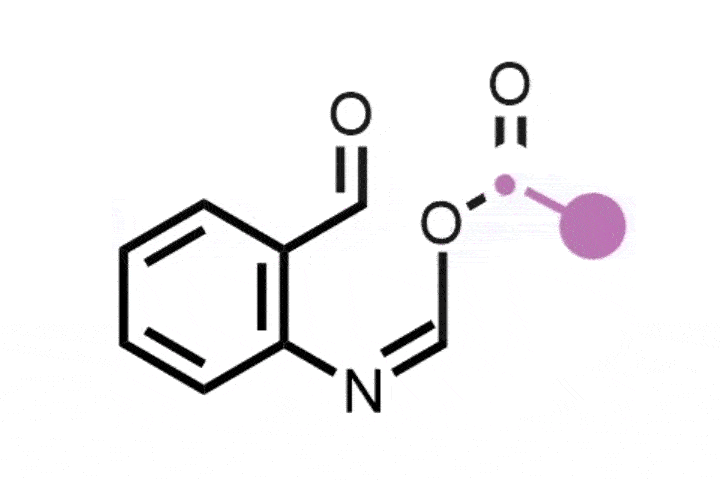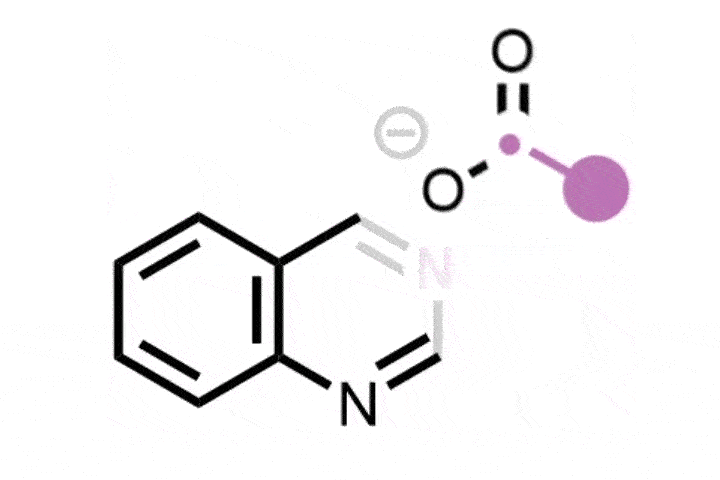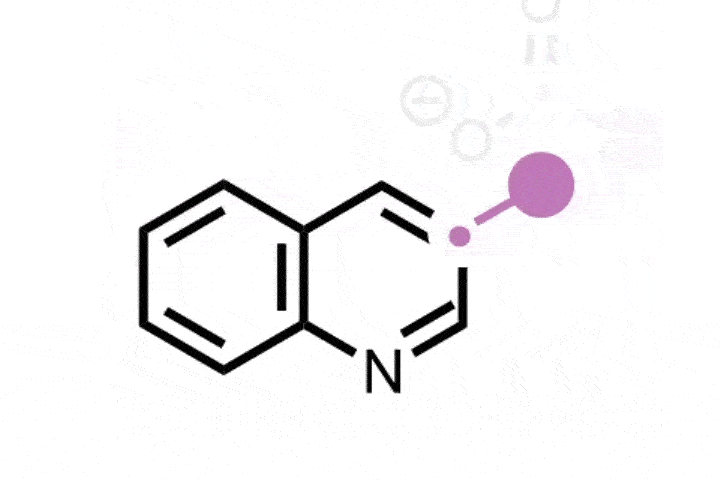
Previously, skeletal editing techniques have focussed on enlarging and reducing the size of rings using single heteroatom deletions or insertions later on in a synthesis. Glorius and Houk’s method takes the field one step further, by inserting a bicyclobutane ring into a thiophene to form an eight-membered ring with a smaller four-membered ring inside of it. The bicyclic structure is analogous to the rigid, complex scaffolds that often appear in natural products and pharmaceuticals. It ‘was a serendipitous discovery, but we quickly realised its importance,’ explains Glorius.
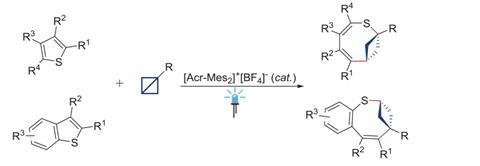
The new method uses a photocatalyst alongside the cheap and mild energy of visible light. Thiophene is activated as the photocatalyst withdraws an electron from the substrate, and bicyclobutane contains strained bonds that attack the activated thiophene. Further rearrangement creates the bicyclic ring system.
The team also tested adding functional groups on both the thiophene and bicyclobutane substrates. With high levels of regioselectivity across a wide range of structural additions the reactions showed high tolerance to a variety of functional groups. Glorius hopes that ‘nitrogen-containing heteroaromatics will be in focus’, as research in the area progresses.
Experts in the field of skeletal editing are impressed with the work. Richmond Sarpong, from the University of California, Berkeley, US, says it combines ‘the power of photoredox chemistry and the increasing availability of bicyclobutanes,’ to supply ‘yet another tool for chemists to radically expand the library of available compounds.’ And Mingji Dai, of Emory University, calls it ‘a remarkable example of skeletal editing’. Following this study, ‘we are much more likely to see more of these “ring-in-a-heterocycle” examples,’ adds Dai.
References
H Wang et al, Science, 2023, 381,75 (DOI: 10.1126/science.adh9737)












No comments yet