Soil search unearths new class of antibiotics
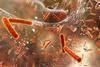
Malacidins have a novel mode of action and can kill MRSA
A new class of antibiotics – named malacidins – has been discovered by researchers genetically screening microbes from crowdsourced soil samples.
The discovery came from a citizen science project – Drugs from dirt – started by Sean Brady of the Rockefeller University, US. The project asked people to send in soil so that the DNA from microbes could be extracted and sequenced.
