Sortase A strategy could see ribosomal products reach further across chemical space
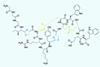
Compound libraries set to benefit from process that harnesses promiscuity of post-translation enzymes
Scientists have simplified a process that harnesses the promiscuity of post-translation enzymes for making and enhancing complex molecules. The team behind the work say it could be developed into a platform technology for generating libraries of compounds with the structural diversity of natural products that are also new to nature.