Study demonstrates how mixtures of isotopologues can store high density information
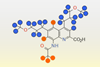
Encoding process works by varying extent of deuteration in an aminoquinoline carboxylic acid
Data can be encoded within mixtures of isotopologues of a carboxylic acid then decoded by analysing those mixtures with mass spectrometry, new research shows. The work illustrates how simple molecules can reliably store high density information.
