Lewis superacid that’s easy to prepare and handle
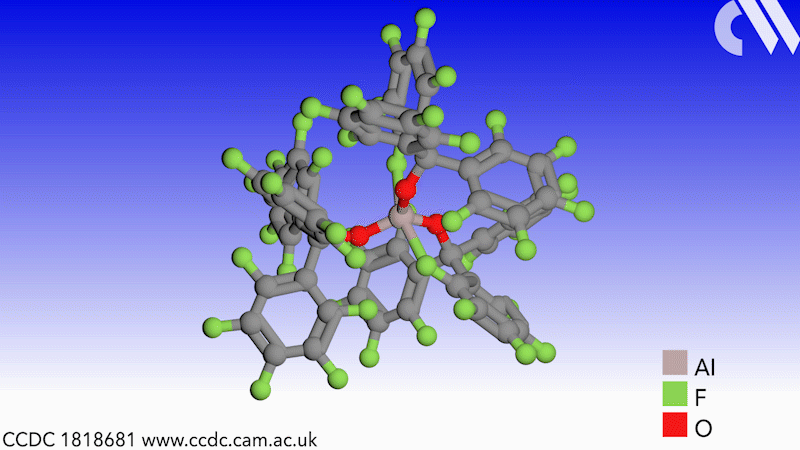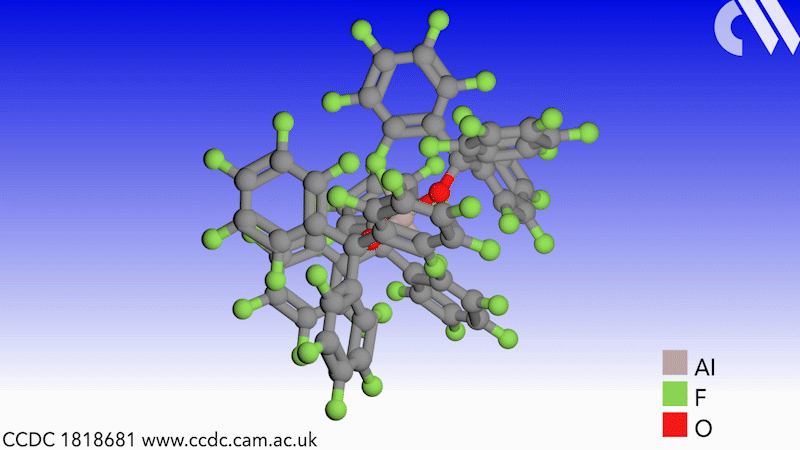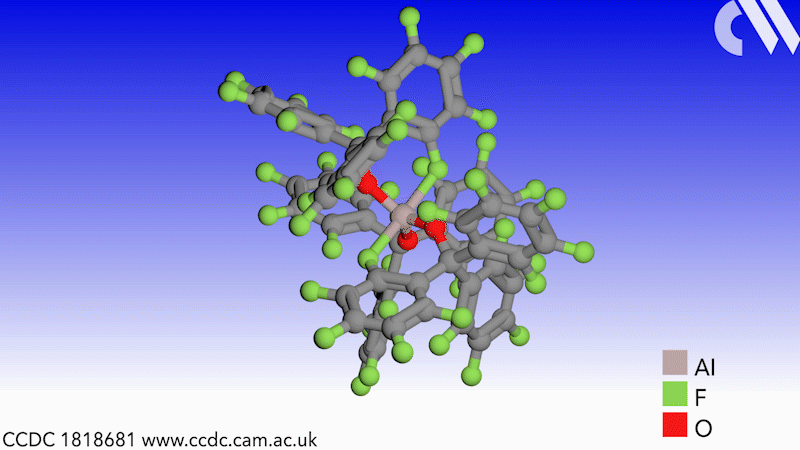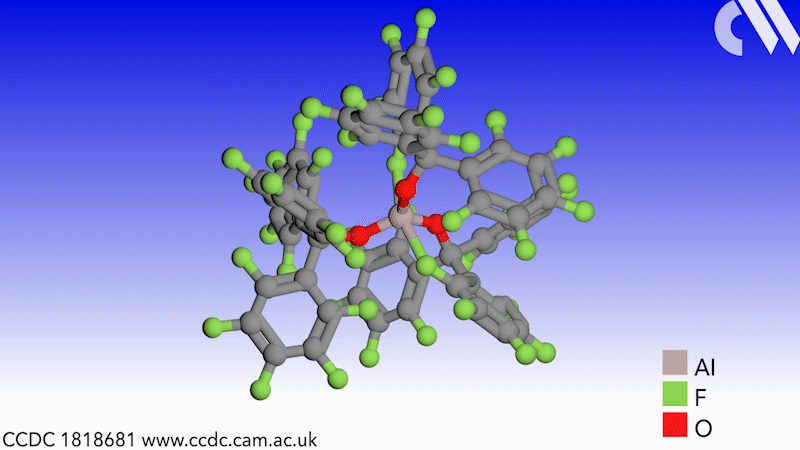
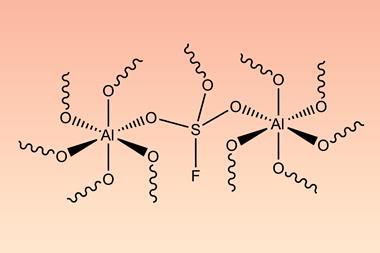
Scientists in Germany and Russia have isolated a new Lewis superacid.1 Previous versions have lacked thermal stability and their related weakly coordinated anions often suffer from severely disordered crystal structures. This new Lewis superacid, Al(OCArF3)3, is stable at temperatures up to 180°C and can form a range of metallates, which can act as weakly coordinated anions.
Lewis acids are vital members of any synthetic chemist’s arsenal and, generally speaking, the stronger the Lewis acid the more efficient it is. Lewis superacids are molecular Lewis acids that are stronger than monomeric SbF3 in the gas phase,2 but their isolation and thermal stability have hindered their applications. The accessibility of this new, versatile series of salts with different countercations could make them useful reagents for catalysis, ionisation, rearrangement and heterolysis reactions and could open up Lewis superacids to a much wider range of chemists.
References
1 J F Kögel et al, Chem. Sci., 2018, DOI: 10.1039/c8sc02981d (This article is open access.)
2 L O Müller et al, Angew. Chem. Int. Ed., 2008, 47, 7659 (DOI: 10.1002/anie.200800783)
![A structure showing that the weakly bonded hydrogen of [P4H]+ sits on the edge of the P4-tetrahedron](https://d2cbg94ubxgsnp.cloudfront.net/Pictures/380x253/7/8/5/138785_c8sc03023e-f5.jpg)








No comments yet