Time to stop mentioning alkyl group inductive effects
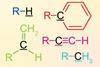
New computational evidence reiterates that alkyl groups are inductively electron-withdrawing and that hyperconjugation dominates
A computational study by researchers in the UK has shown that alkyl groups are inductively electron-withdrawing relative to hydrogen, and not electron-donating as described in some textbooks.
